| Biochemical Mechanisms of Hedgehog Signaling.
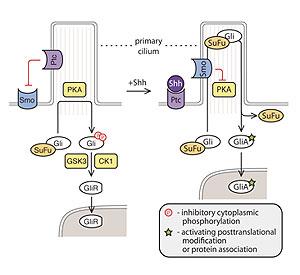
First discovered and studied in Drosophila, the Hedgehog (Hh) signaling pathway regulates cell proliferation, differentiation, and patterning in a range of tissues during animal development. Mutations that cause aberrant Hh signaling lead to birth defects in humans, and genes encoding function as human oncogenes or tumor suppressor genes. Prominent examples of Hh-induced carcinogenesis include medulloblastoma, the most common pediatric brain tumor, and basal cell carcinoma, the most common type of skin cancer, both of which often carry mutations that lead to unchecked Hh signaling. In adults, Hh signaling is thought to regulate the function of tissue stem cells and, under pathological conditions, to sustain tumor stem cells. All things considered, therapeutics and diagnostics based on this pathway hold great promise in cancer and regenerative medicine. Despite its importance, the biochemical mechanisms that drive Hedgehog signaling remain poorly understood in vertebrates. We study three mysterious steps in Hedgehog signaling: 1. The regulation of Smoothened, an oncoprotein and cancer drug target, 2. The mechanism by which Smoothened relays the Hedgehog signals 3. The biochemical mechanism by which the Gli transcription factors Major advances in mammalian Hedgehog signaling have come from the discovery that Hedgehog signaling is orchestrated at the primary cilium. Most proteins in the Hh pathway, including Patched, Smoothened and the Glis, localize in cilia and it is likely that many of the critical reactions in the pathway occur within this specialized compartment. We strive to understand how the carefully choreographed trafficking of Hedgehog pathway proteins at primary cilia drives signal transduction |
The primary cilium is a solitary cell-surface projection found on nearly all cells in our bodies that functions as a major signaling center. Signaling pathways that play critical roles in development and cancer, including Hh, Wnt, and PDGF, have been linked to cilia. Nearly 50 human genetic diseases, called “ciliopathies,” are caused by mutations in gene products required for cilia structure or function. Ciliopathies include some of the most common human genetic diseases, such as polycystic kidney disease. Patients suffering from these syndromes display pleiotropic phenotypes, including polycystic organs, sensory defects, brain malformations, craniofacial abnormalities, skeletal defects, and obesity. Its important to note that these are very common pathological phenotypes in sporadic human maladies as well, suggesting that cilia-based mechanisms will be relevant well beyond inherited human diseases. The >500 proteins that populate the cilia “parts list” represent a completely unexplored therapeutic landscape, both for ciliopathies and for cancer-relevant signaling pathways such as Hh and Wnt. To effectively develop cilia-targeted drugs, we must understand the molecular circuitry (the protein interactions and enzymatic reactions) that link signal transduction to primary cilia. We use Hedgehog signaling as a facile model to reveal general principles of cilia-centered signal transduction. In fact, many ciliopathies show defects in Hedgehog signaling and we are interesting |
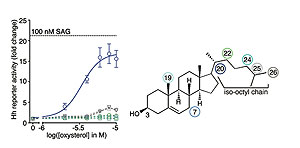
Endogenous Small Molecules in Signal Transduction. While large-scale efforts have been applied to elucidating the functional roles of proteins and nucleic acids, our understanding of endogenous, bio-active small molecules remains primitive. We believe that interactions between proteins or nucleic acids and endogenous small molecules such as lipids or metabolites play a major and largely unexplored regulatory role in the cell. One area of focus is sterols, lipids derived from cholesterol. Sterols influence a broad range of physiological and pathological processes, ranging from lipid metabolism and atherosclerosis to apoptosis, immunity and cancer susceptibility. In many of these cases, the specific mechanisms and molecular pathways by which sterols exert their effects on cells remain unknown. We are developing broadly applicable, next-generation methods to visualize the distribution and trafficking of sterols in cells and animals and methods to comprehensively map sites in proteins that interact with these important lipids. We strive to uncover novel regulatory mechanisms that mediate the effects of sterols |